One More Shot
Cincinnati Children’s Hospital has begun trials studying the COVID-19 vaccine in children ages 12 to 15 years old.
May 28, 2021
STORY MIA HILKOWITZ | PHOTOGRAPHY RILEY HIGGINS | INFOGRAPHIC CAITLIN O’DONNELL
Since the COVID-19 vaccine was approved for emergency authorization use in Dec. 2020, biomedical companies like Pfizer and Moderna have been continually trying to lower age restrictions in hopes of increasing vaccinations worldwide. Cincinnati Children’s Hospital has worked to reach authorization for a younger age group: 12 to 15 year olds. The nationally recognized hospital is running trials to test the Pfizer COVID-19 vaccine on participants in this young population to expand the number of citizens eligible for the vaccine.
Professor of Pediatrics in the Division of Infectious Diseases at Cincinnati Children’s Hospital Robert Frenck has been directing these trials through the Gamble Center for Research. Frenck says that his department has been working for months to test and develop the vaccine.
“When COVID-19 started, [the National Institute of Health] contacted us to start working with them on the vaccine,” Frenck told Spark. “Pfizer [also] talked with us to ask us to help them with their vaccine testing.”
Cincinnati Children’s Hospital has participated in numerous other vaccine trials this year, first focusing on the adult age group then transitioning to a younger population. According to Frenck, about 1,400 people have enrolled in the different Cincinnati Children’s trials. Currently there are 350 12-15 year olds in the ongoing trial. Frenck described the detailed process that’s used to study the COVID-19 vaccine.
“First you have to assess that you have the resources you need such as the number of study personnel, that we have enough space to be able to store the vaccine, and that we have the room to be able to have people come in,” Frenck said. “Then we have a protocol which says how we’re going to do this study, and the protocol is then submitted to a review body called the Institutional Review Board.”
The Institutional Review Board is a Food and Drug Administration (FDA) organization that reviews and monitors biomedical research involving humans. The Institutional Review Board must approve an organization’s protocol before trials can begin. After approval, Cincinnati Children’s started the search for its young participants.
“We put out advertisements letting people know about the study. They come in and talk to us and see if they’d be interested in participating,” Frenck said. “If people come in and say yes, that they would like to know more, we review with them the informed consent document.”
An “Informed Consent Document” is the form used in a clinical trial that explains to potential participants the risks and benefits of a study, as well as the rights and responsibilities of participants and researchers involved. According to the FDA, an Informed Consent Document helps to protect the rights of participants in a study. Following the review and signing of this document, participants undergo a background check and begin the trial.
“[First] we would get some blood from [the participants] because you need to know what their antibody level and immune response is before they have a vaccine,” Frenck said. “Then they get a dose of the vaccine and come back in three weeks. Then [they get] another blood test to see how the immune response is so far and get another dose of the vaccine. Then they come back and we get another blood sample and see how the second dose affected their immune response.”
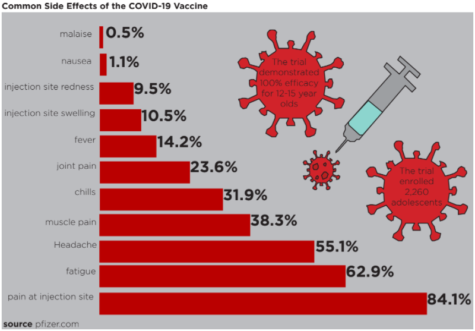
Following the initial trial, researchers follow the participants over time to monitor symptoms and side effects and to make sure the vaccine is appropriately preventing infections. According to a Cincinnati Children’s study released Mar. 31, the vaccine was 100% effective in preventing COVID-19 infection in children aged 12 to 15. On May 25, the FDA approved emergency use for those 12 to 15-year-olds. Frenck believes that the vaccine will be widely available for the age group before the start of the 2021-22 school year.
Cincinnati Children’s Hospital Associate Division Director of Primary Care and Pediatrics Mary Burkhardt thinks that these types of studies on COVID-19 are beneficial to the pediatric field.
“At the beginning of the pandemic, COVID-19 was new to all of us,” Burkhardt told Spark. “We were not sure who would be impacted and what the short and long term implications of infection were. Often infectious diseases affect the young and old most severely, so it’s great to learn and better understand that young people did not have the same severity of illness as many older people.”
According to the American Academy of Pediatrics, as of May 6, over 3.85 million children in the U.S. had tested positive for COVID-19 since Mar. 2020. Only 0.1-1.9% of child COVID-19 cases resulted in hospitalization. The Centers for Disease Control and Prevention (CDC) reported that the highest rate of hospitalization is 12% among those 85-years-old and up.
“Generally, most children have more mild effects of COVID-19,” Burkhardt said. “Many have symptoms for several days, but they make a full recovery. We have seen that other children are affected more significantly, often children who have other underlying illnesses.”
Director of Infectious Diseases at Cincinnati Children’s Hospital Paul Spearman said that coronaviruses are not necessarily new illnesses in the medical field. For instance, the first human coronavirus was discovered in the mid 1960s, and now scientists have identified seven different strands of the virus.
“We see coronaviruses that don’t cause nearly this degree of illness,” Spearman told Spark. “They are commonly seen in kids and generally just cause an upper respiratory infection.”
Similar to adults, common symptoms of COVID-19 in children include fever, fatigue, headaches, coughing, loss of taste or smell, sore throat, shortness of breath, difficulty breathing, abdominal pain, and nausea. Spearman, who initially ran an Human Immunodeficiency Virus (HIV) Infection lab, has transformed his department to begin studying COVID-19 and assist in the testing and development of the vaccine.
“Our group has been involved in numerous COVID-19 vaccine trials during the last year,” Spearman said. “We turn a lot of resources to the problem of the moment. There needed to be such a large effort. And then there are people who have worked on coronaviruses for years who can do more of the complex experiments.”
An Apr. 2021 Reuters report found that 55% of U.S. parents said they were interested in getting their child vaccinated against COVID-19. Spearman said that the concerns of the other 45% should still be addressed.
“There is a substantial minority of the population who have reservations about vaccines for a number of reasons and I think that’s very important to recognize,” Spearman said. “It’s important to get the right information out so that everyone can evaluate for themselves the safety track records of the vaccines and how well they work and make a decision to hopefully get the vaccine. It’s very important that there is a high uptake of the vaccine so that we can get out of this pandemic and get out of room and our Zoom calls.”
Spearman believes that it is important for parents to receive accurate information regarding the vaccine, and he offers a message to those with hesitance.
“The first thing I would tell them is that I would give it to my kids,” Spearman said. “My kids are adults now and when they were younger we always vaccinated them. We know how important it is for kids to be protected from things like influenza, and for COVID-19 it’s, at the moment, even more important that they get protected.”